Architecture of Chemical System Component
The need for the Chemical System component stemmed from the high-level architecture details described Architecture of Chemist.
Considerations for the Chemical System Component
- System building
The overall point of the chemical system component is to provide an object- oriented DSL which the user can use to build up the chemical system they want to model. The component is focused on specifying the system, not the specifics of the physics model.
There is admittedly a fine-line between specifying the system and specifying the physics model. For example, one may reasonably argue that the specification of the atomic basis set is a means of specifying the electrons on an atom. Ultimately the delimitation between chemical system and physics model is a convention and meant to parallel actual usage.
- Fundamental particles
An object-oriented DSL for building up a chemical system should enable the user to combine fundamental particles (for the moment defined as nuclei and electrons) to build up the system.
Requiring the user to say actually add say 92
Electronobjects to anAtomobject in order to create uranium is overkill. While conceptually this is what the user is doing, user-friendly APIs should exist.Exactly what defines a fundamental particle should be allowed to change. For example, extensions which actually resolve the nucleon structure of the nucleus are conceivable. Similarly excitons
- Fields
Particularly when modeling chemical systems in condensed phase there is a tradition to think of parts of the system as being a field (usually an electrostatic field) rather than their true chemical identity. For example, one may replace the solvent with a field meant simulate the bulk.
Interactions with light could fall under this category two.
- Quantum vs. classical
The chemical system component should be capable of describing which parts of the system are treated classically and which are treated quantum mechanically.
Concepts like molecules look different depending on the physics used to model them. For example, many quantum chemistry methods assume a molecule is a set of nuclei (modeled as classical point charges) interacting with a set of quantum mechanical electrons; notably this means we can not uniquely associate any particular electron with a particular nucleus (as would be required to define atoms). Conversely, a classical molecule does assign electrons to nuclei (most force fields do this by assigning net charges).
Out of Scope
- Basis Sets
In an attempt to decouple how quantum mechanical objects are described from identifying which objects are quantum mechanical, we opt for the basis sets to be modeled separately.
Overview of the Chemical System Component
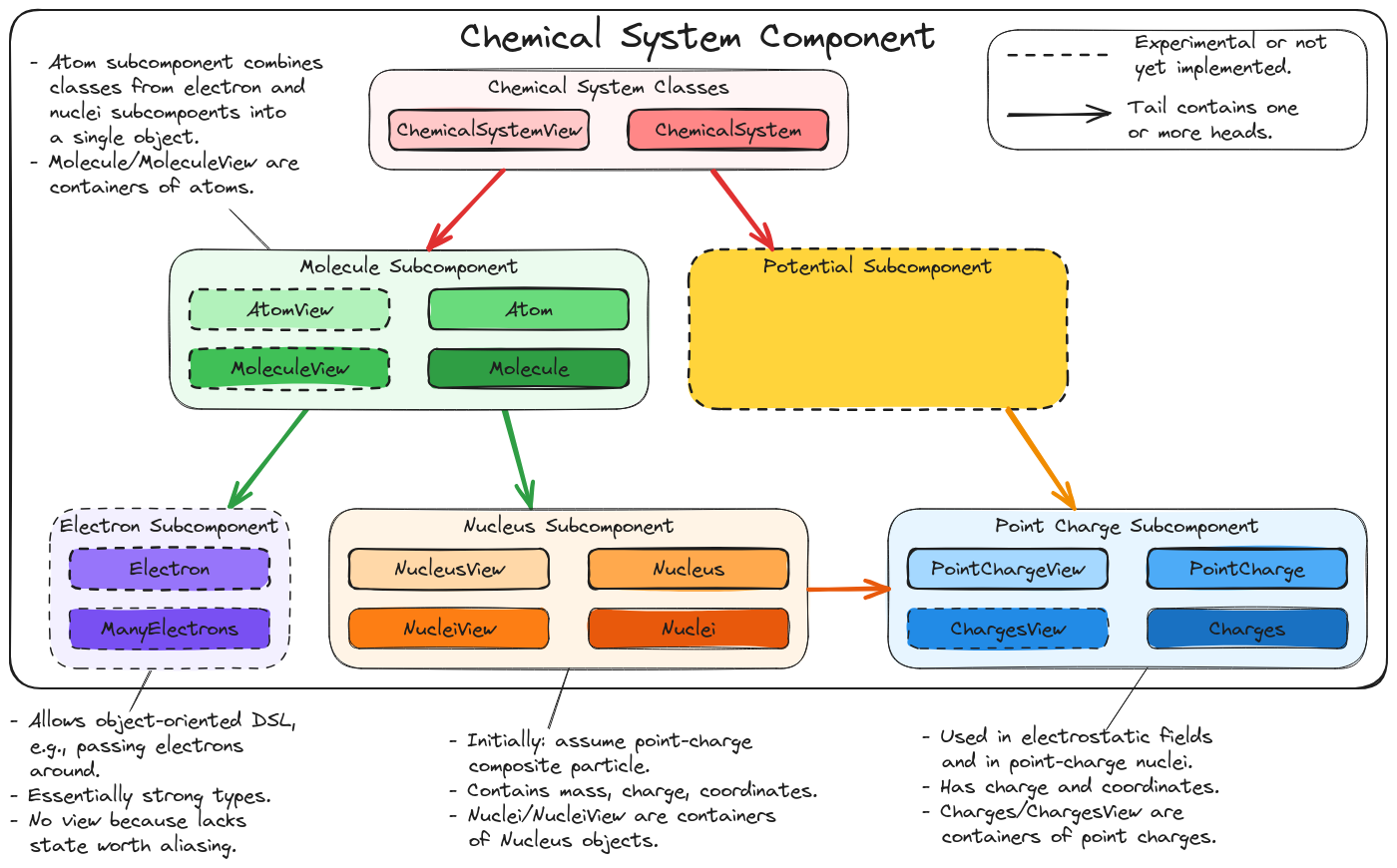
Fig. 3 Major subcomponents and classes of the Chemist’s Chemical System component.
Nucleus Subcomponent
Main discussion: Designing the Nucleus Component.
Molecule Subcomponent
Main discussion: Molecule Design.
Chemical System Classes
Main discussion: Chemical System Design